The world's first lightweight, intelligent fatty liver screening device, a portable solution that measures the presence and condition of fatty liver within 30 seconds through non-invasive ultrasound diagnosis.
FattaLab® 지방간 진단 기기
Eieling Technology Limited
Fatty liver disease often presents without initial symptoms but can be fatal if it progresses.
Existing diagnoses rely on ultrasound or blood tests in hospitals, which are costly, time-consuming, and difficult to undergo regularly.
The increasing obese population leads to a rise in individuals at risk of fatty liver disease. However, the lack of self-management methods results in poor early detection.
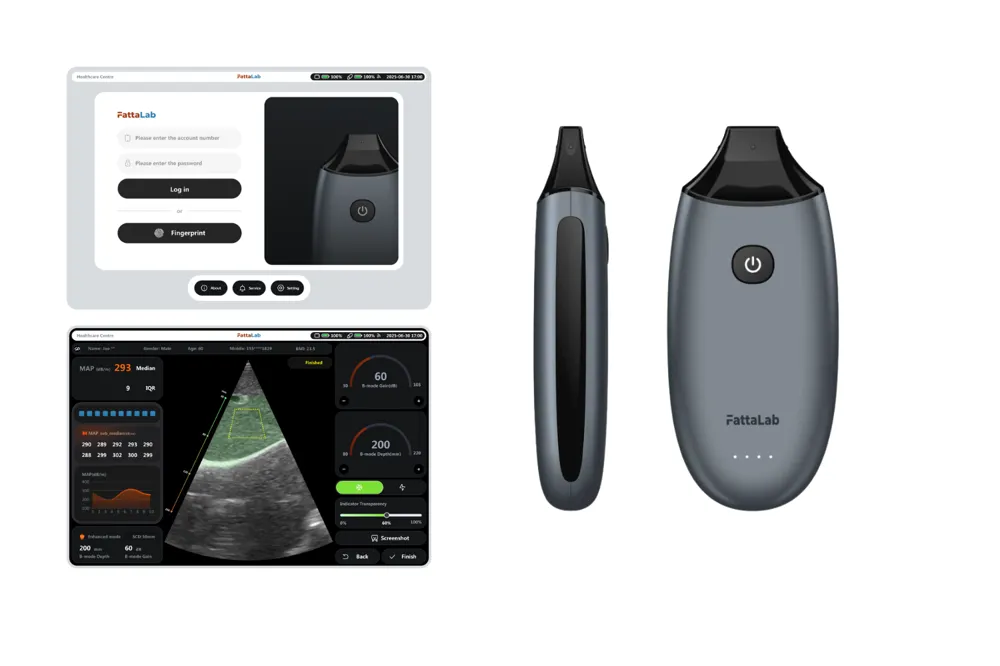
FattaLab is a palm-sized (approximately 120g) ultrasound device that provides intrahepatic fat data within 30 seconds by simply placing it on the abdomen and pressing a single button [16].
It enhances accuracy with an AI-based liver tissue recognition algorithm and accurately measures obese patients with thick subcutaneous fat using dual-domain signal processing technology [17].
With a battery life of over 2 hours, it can be used for on-site check-ups, and an automatic report is generated through a smartphone app, making the results easy for the general public to understand.
The implementation of performance comparable to existing hospital equipment in a portable device, and the automation that allows anyone to use it without training, are innovative.
Hospitals, medical examination centers, and other medical institutions (B2B), as well as corporate check-up programs and public health centers, are the main buyers.
In the long term, healthcare companies or individuals (B2C) can also access it in the form of purchase or service use.
There is high global demand in the chronic disease management market.
As a Hong Kong startup, it is seeking to enter the global medical device market beyond China and Asia [18], and can be expanded into remote healthcare kits or home health devices once it obtains FDA and other national approvals.
Additionally, there is room for development into an integrated liver health solution, such as monitoring hepatitis and cirrhosis.
It is evaluated as a case with both technical completeness and usefulness.
Clinical data-based reliability has been secured with the support of the Hong Kong Science Park and academia (PolyU) [19], and it has been well-received in the Digital Health category at CES [18].
However, there is a realistic point that regulatory approval and acceptance by the medical community are the next challenges in the commercialization of medical devices.
🔥 High market potential / Business connection possible (High expectations for commercialization due to definite medical demand for non-invasive early diagnosis)
The award list data is based on the official CES 2026 website, and detailed analysis content is produced by USLab.ai. For content modification requests or inquiries, please contact contact@uslab.ai. Free to use with source attribution (USLab.ai) (CC BY)