EXoPERT's Exosome-SERS-AI multi-cancer early diagnosis platform is an innovative solution that combines exosome-based liquid biopsy and AI technology to rapidly and accurately diagnose multiple cancers, including early-stage cancers.
Exosome-SERS-AI 다중 암 조기 진단(MCED) 플랫폼
EXoPERT
Existing early cancer diagnosis relies on blood ctDNA tests or NGS genomic analysis, which are costly and time-consuming. In the case of early-stage cancers, detection sensitivity is limited. In addition, reliance on single biomarkers or result variations depending on proficiency makes it difficult to test for multiple types of cancer at once.
As a result, patients often miss the opportunity for early cancer detection or experience delays in diagnosis, leading to missed treatment windows. The global early cancer diagnosis market is expected to grow to tens of trillions of Korean won in the future, but the absence of fast and accurate multi-cancer diagnostic technology to support this growth remains a challenge.
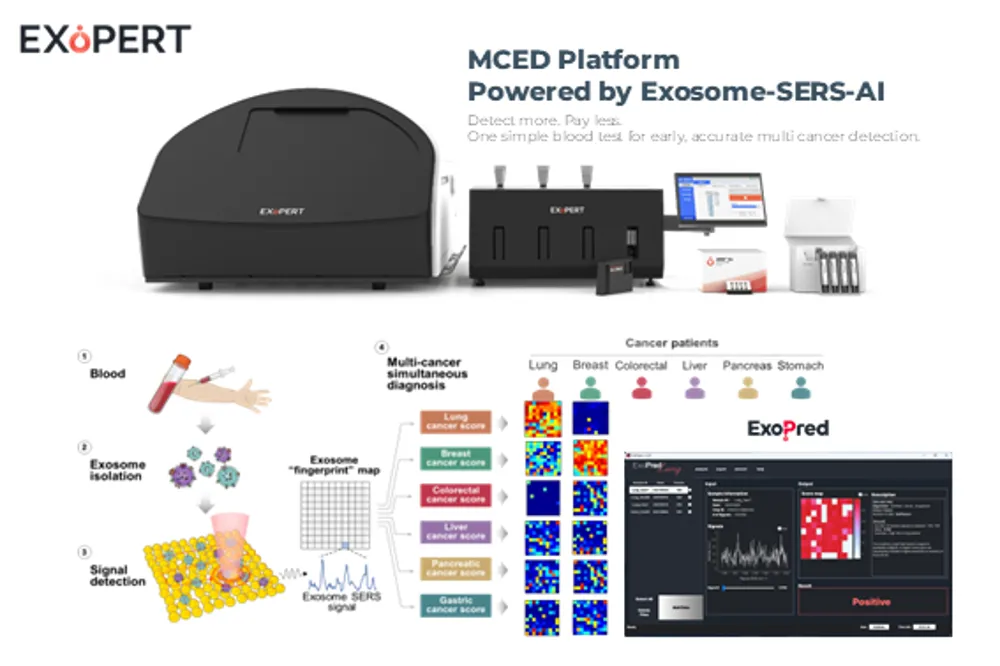
The EXoPERT platform is innovative in that it combines exosome-based liquid biopsy with SERS (Surface-Enhanced Raman Spectroscopy) technology and AI algorithms to simultaneously diagnose multiple cancers. In particular, exosomes in the blood are extracellular vesicles that are secreted in large quantities even in early-stage cancers and contain molecular information from cancer cells. EXoPERT analyzes these exosomes with high sensitivity to significantly improve the accuracy and efficiency of existing methods.
As a result, it achieves excellent performance with 90.2% sensitivity and 94.4% specificity (based on early-stage cancer), allowing multiple cancers to be screened at once within 5 minutes. In addition, the system iscomposed of a 3-step automated workflow (exosome separation → Raman signal capture → AI interpretation)enabling diagnosis with a cost reduction of over 50% without the need for specialized personnel, and providing stable results without inter-operator variation through disposable cartridge-based automation.
Hospitals and medical examination centers (B2B)are the key customers. If the cancer screening departments of large general hospitals or national cancer screening programs adopt this platform, multiple cancers can be screened early with a single drop of patient blood.
In addition, it can be used for large-scale population screening by government health authorities (B2G), and in the long term, *primary care institutions (clinics)* or specialized screening startups can purchase devices and kits to provide services. Currently, it is in the clinical trial and commercialization preparation stage with research collaboration institutions (Johns Hopkins University, MD Anderson Cancer Center, etc.).
Technically, since it is a multi-biomarker platform for blood exosomes, it can be expanded to include more target cancers or to diagnose diseases other than cancer (such as Alzheimer's) in the future. However, obtaining medical device approval and large-scale clinical validation are essential, and these are currently being accelerated in Korea and the United States.
From a regulatory perspective, approval of in vitro diagnostic devices is key, and the FDA approval (SaMD) process for AI diagnostic algorithms is also necessary. EXoPERT is already building industry recognition by receiving US JLABS, Korean innovative medical device designation, etc., and is seeking to enter the global market, and has the potential to become a standard platform for early cancer screening in the long term.
It is recognized on the international stage for "the innovation of multi-cancer early diagnosis combining exosomes, AI, and Raman analysis" and has been awarded the CES Innovation Award. Industry journals are praising it as "a new paradigm that overcomes the limitations of existing technology," and in particular, they evaluate that the technological ripple effect is significant in that it simultaneously diagnoses 5 types of early-stage cancer.
However, it is currently in the pre-commercialization stage, and despite winning the CES award, market verification has not yet been achieved. Although the technological completeness is high, there are still hurdles to overcome, such as clinical trials, approvals, and insurance coverage, so investors believe that "although it has received attention at CES, it will take time to enter the actual medical market."
🧪 R&D and concept verification stage (the technology is innovative, but clinical validation and commercialization hurdles must be overcome).
The award list data is based on the official CES 2026 website, and detailed analysis content is produced by USLab.ai. For content modification requests or inquiries, please contact contact@uslab.ai. Free to use with source attribution (USLab.ai) (CC BY)