Wis Medical's Tedream™ is an innovative home sleep test solution that conveniently measures 7 bio-signals at home and diagnoses sleep apnea with AI analysis.
Tedream™
Wis Medical
Sleep apnea is associated with serious complications such as dementia, but accurate diagnosis requires an overnight stay in a hospital and a PSG test involving the attachment of dozens of electrodes. This situation, coupled with barriers such as months of waiting for appointments, test discomfort, and costs (hundreds of thousands of won), prevents most potential patients from getting tested.
Existing smartwatches' sleep measurements have limitations in accurately providing breathing or oxygen saturation information, making professional screening difficult. In other words, the problem was that there was no easy way to detect sleep disorders without going to the hospital.
Tedream aims to solve this by pursuing both medical-grade measurement accuracy and user convenience.
##
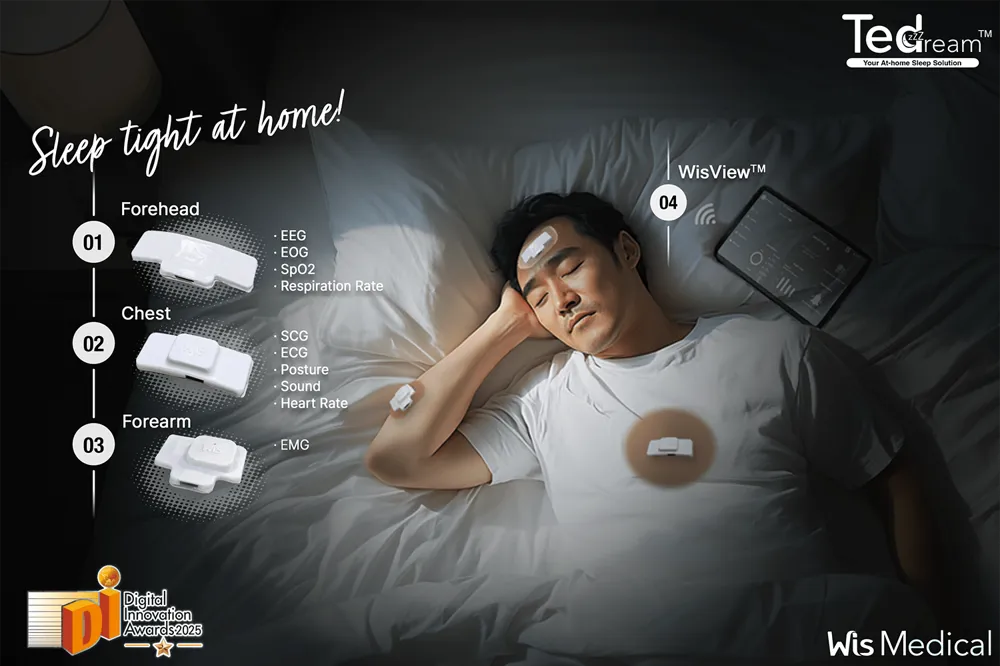
Tedream is capable of comprehensive biosignal measurement, so it can be called "at-home polysomnography".The forehead patch measures brain waves (EEG) and eye movements, the chest patch measures electrocardiogram (ECG) and respiratory movements, and the arm patch measuresoxygen saturation (SpO₂) and muscle tension (EMG).are measured.
With ultra-small sensors and wireless communication, there are no cords or machines connected to the body during sleep, allowing for free sleep, including tossing and turning. The collected data is transmitted to the cloud via smartphone, where an AI algorithm instantly analyzes it to calculate apnea index, heart rate variability, snoring patterns, etc.
In particular, AI, which detects sleep apnea with high accuracy from multiple signals, is key, and showed a high correlation with hospital PSG in clinical trials. In addition, Qi wireless charging and a patch reuse structure minimize disposable consumables, making it economical.
While most competing solutions use only a finger oxygen saturation sensor, Tedream is the first/best-in-class home solution in that it comprehensively measures 7 biosignals at once.
Individuals (B2C) who are concerned about snoring/apneaare the main customers. For example, a middle-aged man who wants to check if he has sleep apnea at the recommendation of his spouse, or an office worker who is anxious because of irregular sleep patterns, will purchase the device directly.
After the fee is applied,hospitals or insurance companies (B2B)may also use it. Sleep clinics can send patients home with the device during the initial consultation to secure patient data, or insurance companies can provide it to subscribers as a health management service. In addition, a model is possible in which corporate welfare programs or health checkup centers lend the device to employees/examinees to be tested at home.
Initially, sales will begin based on doctor's prescriptions and will later expand to general distribution channels such as pharmacies (company press release).
##
The potential market is very large, with over 1 billion snorers worldwide (a significant number of whom are estimated to have apnea). It can be used in the global medical device market once regulatory approvals are obtained from each country's regulatory authorities, and is currently preparing for US FDA approval (News1 report).
The vast amount of sleep big data accumulated by cloud AI can create added value in the future by developing new sleep health indicators or personalized treatment recommendation algorithms.
Furthermore, the technology of measuring key signals with only patches without wearables can be applied to other disease monitoring (e.g., arrhythmia monitoring patches), expanding the possibility of becoming a healthcare platform company.
The challenge is to secure trust through medical device approval and acceptance by the medical professional community. However, as it was developed with the support of local governments such as Yongin City, policy support is also expected. It is evaluated that if the price is lowered and the usage is made easier, the era in which this device is included in general health checkup items in the future is also possible.
##
The reaction from domestic and foreign experts is very positive. It has attracted attention in the sleep medicine field to the point where it has been called *"the holy grail of home polysomnography."*
After being introduced to the international stage with the CES Innovation Award, medical startup officials commented that *"the sophisticated use of AI is impressive, and the sleep medicine landscape will change when it is commercialized."* In domestic news reports such as News1, government officials mentioned that it is *"a promising technology that will also work in the North American market."*
However, from the perspective of general consumers, there are also indications that *"the idea of doing an uncomfortable PSG test at home is still unfamiliar"* even though this product is innovative, and market education is needed. It is also mentioned that the timing of the launch is unclear because regulatory approval procedures such as the FDA remain.
Overall,*"If accuracy is verified, demand will explode"*is expected, along with*"Clinical verification and approval are key"*is a mixed realistic assessment.
##
🧪 R&D and concept verification stage (Although it has excellent innovation and great market potential, it can still be seen as a stage to prove the technology concept because there are hurdles to overcome, such as medical regulatory approval and large-scale clinical verification.)
## Demo Video
Tedream Patch Attachment and Sleep Data Check Process – YouTube: "Tedream Home Sleep Test Demo"
The award list data is based on the official CES 2026 website, and detailed analysis content is produced by USLab.ai. For content modification requests or inquiries, please contact contact@uslab.ai. Free to use with source attribution (USLab.ai) (CC BY)